Biomimetic Somatosensory Feedback through Intracortical Microstimulation
Sliman Bensmaia, Department of Organismal Biology and Anatomy
University of Chicago
Warren Grill, Department of Biomedical Engineering
Duke University
People: Joseph Sombeck, Kyle Blum, Raeed Chowdhury, and Juliet Heye
Spinal cord injury causes both paralysis and loss of sensation from the limbs. The past 15 years have seen remarkable advances in “Brain Computer Interfaces” (BCIs) that allow paralyzed persons to move anthropomorphic limbs using signals recorded directly from their brains (Hochberg et al., 2012; Collinger et al., 2013). However, these movements remain slow, clumsy, and effortful, looking remarkably like those of individuals who have lost sensation from their arms due to peripheral neuropathy. Quite recently, a great deal of interest has developed in using intracortical microstimulation (ICMS) to restore lost somatosensation and to improve the use of brain-controlled prostheses (O’Doherty et al., 2011; Flesher et al., 2016). Of particular interest to our group is proprioception, the sense of limb position and movement.
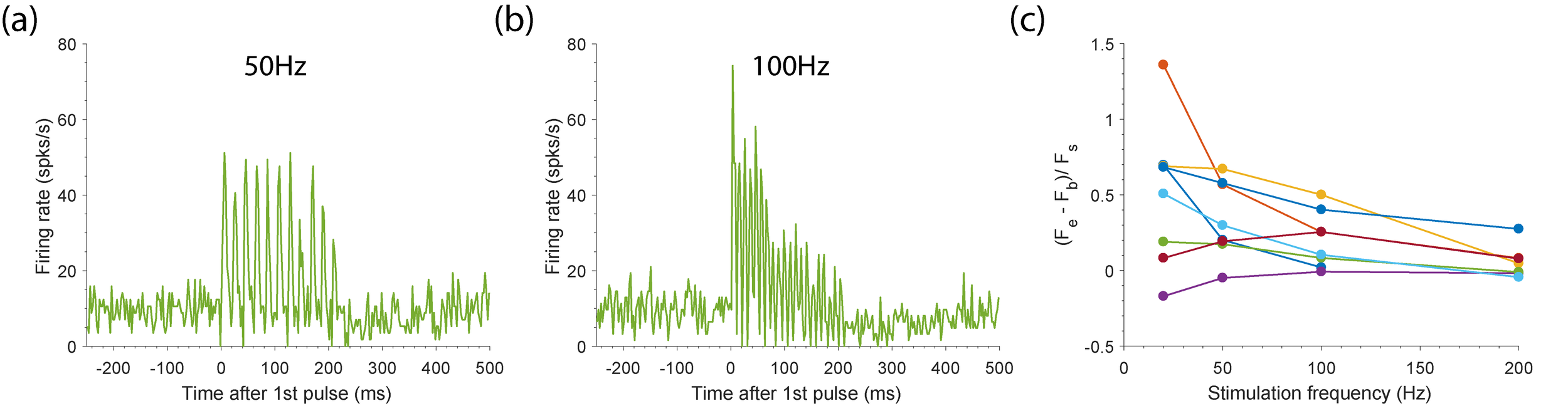
Patients without proprioception make their largest errors during rapid movements, in part, because the slow speed of visual feedback limits correction of these movements (Ghez et al. 1990). Movement planning based on current limb state is also compromised. A proprioceptive interface, then, needs to provide accurate, rapid state information. Earlier work has had some success in training monkeys to recognize arbitrary patterns of stimulation as proxies for touch (O’Doherty et al., 2011; Klaes et al., 2014) and proprioception (Dadarlat et al., 2015). In this project, we seek to develop more nearly biomimetic stimulus paradigms that may require less learning on the part of the user. Using a biophysical model of the cortical response to stimulation (Aberra et al., 2018), our colleagues at Duke University, led by Dr. Warren Grill, have developed model-optimized mappings between limb state and the patterns of ICMS required to evoke S1 activation that mimics that of natural inputs. We are working to validate this model by comparing modeled ICMS-evoked activity to that recorded in S1 using novel hardware that allows for recording at very short latency after stimulation. We have recorded the response in S1 on the stimulated electrode to trains of varying frequencies (Fig. STIM_a-b). In this example, each pulse in the 50Hz train evoked a similar response in the neuron, while the evoked response diminished over the course of the 100Hz train. In general, some neurons have a maintained response throughout an extended stimulus train, while others do not, especially when stimulating above 100Hz (Fig. STIM_c). We observed a similar effect in modelled neurons. We are currently comparing other characteristics of the response to stimulation to further validate the model.
Fig. STIM: Evoked response to trains of stimulation. The average neural activity recorded on an example channel stimulated with 200ms long trains consisting of cathodic-first pulses is shows for (a) 50Hz and (b) 100Hz stimulation. Stimulation starts at 0ms and ends at 200ms. Recorded spikes were binned with 2.5ms bins. (c) The slope of the line fit to the firing rate throughout the train for different train frequencies is shown for a subset of neurons (colors).
Sliman Bensmaia’s group at the University of Chicago has used these methods to develop algorithms to map touch -- its location on the hand and the time course of contact -- to single-electrode stimulation. They have applied this approach successfully in both monkeys and humans (Tabot et al., 2013; Flesher et al., 2016; Saal et al., 2017). Bensmaia’s group and our group are working to develop the more complex spatiotemporal patterns of stimulation that will be necessary to recreate the multi-contact patterns of tactile cues resulting from object manipulation, as well as the complex patterns of activity that accompany limb movement in proprioceptive areas of cortex, respectively. In earlier work in our lab, we stimulated on groups of four electrodes, each with similar response properties, and successfully generated a simple percept of limb movement in a single monkey (Tomlinson, Miller, 2016). Unfortunately, we were unable to replicate this result in subsequent monkeys. We have since begun to study the cortical representation of limb state in more detail, and to investigate the effects of cortical stimulation with many more electrodes.
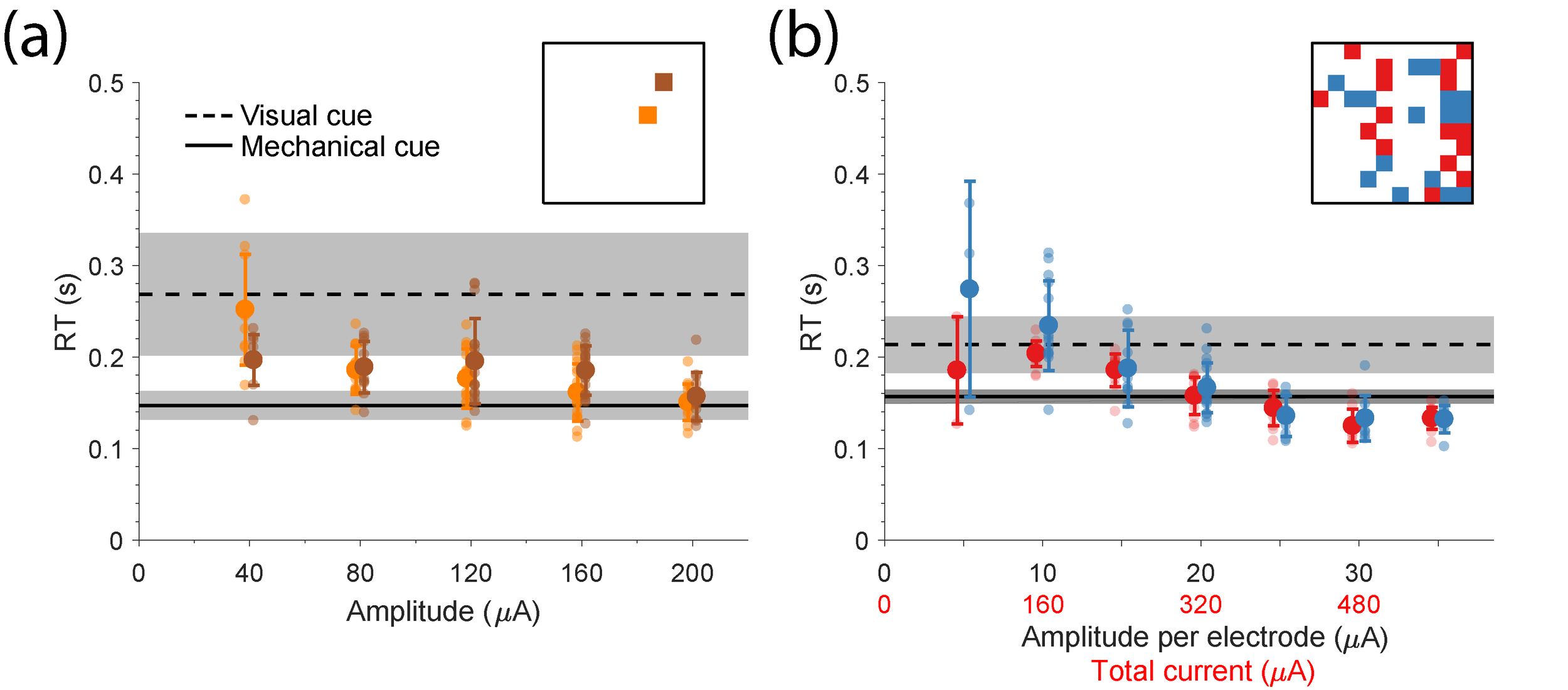
In prior experiments investigating the potential for ICMS to be used to replace somatosensation, monkeys have been unable to respond as quickly to ICMS as to natural cues, casting doubt on the ability of ICMS to replace proprioception (London et al., 2008; Godlove et al., 2014). To investigate this question, we used a reaction time (RT) paradigm to compare the latency of ICMS applied through multi-electrode arrays implanted in area 2 of somatosensory cortex (S1) to that of perturbations applied to the hand and to visual cues. Consistent with earlier studies, we found that ICMS applied through a single electrode typically resulted in RTs that were slower than limb perturbations, even with very large currents (Fig. RT_a). On the other hand, stimulation through many electrodes could produce reaction times as fast or even faster than limb perturbations (Fig. RT_b). This result implies that the complex spatiotemporal patterns of ICMS that may be necessary to create meaningful patterns of cortical activity, may also provide a very rapid means to write in somatosensory information to the central nervous system.
Fig. RT: Reaction time to single- and multi-electrode stimulation. (a) The reaction time (RT) to single electrode stimulation for two different electrodes is shown for a wide range of amplitudes. Large circles represent the mean RT for each amplitude. Small circles show the RT for single trials. Black horizontal solid line shows the RT to the mechanical cue during the corresponding session. Black horizontal dashed line shows the RT to the visual cue All error bars show standard deviation. Inset denotes the position of the two electrodes on the array. (b) The RT to multi-electrode stimulation for two different patterns is shown for a wide range of amplitudes.
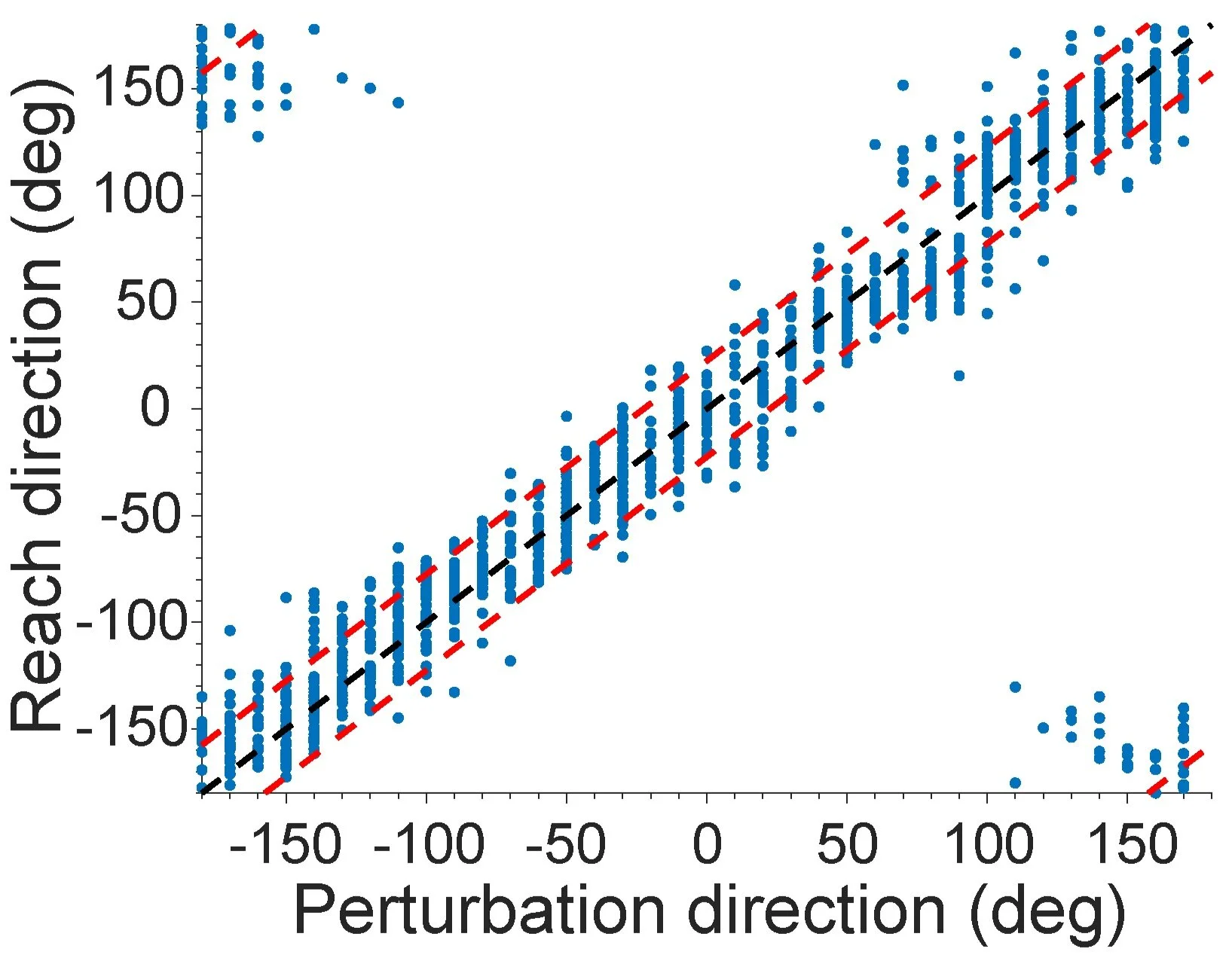
In order to extend these results, we plan to use multi-electrode ICMS to produce naturalistic patterns of neuronal activity in S1 of monkeys, hypothesizing that these patterns will require less training to interpret than arbitrary ones. To evaluate the maps, we will train monkeys to report the direction of brief force bumps applied to their hands (Fig. RR). After training, we will replace the actual bumps with virtual bumps created by patterned ICMS using the maps described above, again asking the monkeys to report their perceived sense of the direction of the perturbation. We expect that the model-optimized maps will result in predictable reported directions, implying that a sensation of limb movement in the reported direction was elicited. The ultimate goal is to demonstrate that this model-optimized, biomimetic feedback is informative and easy to learn, it should form the basis for robust, scalable, somatosensory feedback for BCIs.
Fig. RR: Reported direction matches bump direction. The reach direction made in response to bumps in different directions is shown. Each blue dot represents the reach direction made during a single trial. The black dashed line represents unity, and the red dashed lines represent the error tolerance within which the monkey was rewarded.
Citations:
O'Doherty, J. E., M. A. Lebedev, P. J. Ifft, K. Z. Zhuang, S. Shokur, H. Bleuler and M. A. L. Nicolelis (2011). "Active tactile exploration using a brain-machine-brain interface." Nature 479(7372): 228-231.
Klaes, C., Y. Shi, S. Kellis, J. Minxha, B. Revechkis and R. A. Andersen (2014). "A cognitive neuroprosthetic that uses cortical stimulation for somatosensory feedback." Journal of Neural Engineering 11(5): 056024.
Dadarlat, M. C., J. E. O'Doherty and P. N. Sabes (2015). "A learning-based approach to artificial sensory feedback leads to optimal integration." Nat Neurosci 18(1): 138-144.
Aberra, A. S., A. V. Peterchev and W. M. Grill (2018). "Biophysically realistic neuron models for simulation of cortical stimulation." Journal of neural engineering 15(6): 066023.
Saal, H. P., B. P. Delhaye, B. C. Rayhaun and S. J. Bensmaia (2017). "Simulating tactile signals from the whole hand with millisecond precision." Proceedings of the National Academy of Sciences 114(28): E5693-E5702.
Tabot, G. A., J. F. Dammann, J. A. Berg, F. V. Tenore, J. L. Boback, R. J. Vogelstein and S. J. Bensmaia (2013). "Restoring the sense of touch with a prosthetic hand through a brain interface." Proceedings of the National Academy of Sciences 110(45): 18279-18284.
Tomlinson, T. and L. E. Miller (2016). Toward a proprioceptive neural interface that mimics natural cortical activity. Progress in Motor Control: Theories and Translations. J. Laczko and M. L. Latash, Springer.
London, B. M., L. R. Jordan, C. R. Jackson and L. E. Miller (2008). "Electrical stimulation of the proprioceptive cortex (area 3a) used to instruct a behaving monkey." IEEE Trans Neural Syst Rehabil Eng 16(1): 32-36.
Godlove, J. M., Whaite, E. O., & Batista, A. P. (2014). Comparing temporal aspects of visual, tactile, and microstimulation feedback for motor control. Journal of neural engineering, 11(4), 046025.
Hochberg, Leigh R., et al. "Reach and grasp by people with tetraplegia using a neurally controlled robotic arm." Nature485.7398 (2012): 372.
Collinger, Jennifer L., et al. "High-performance neuroprosthetic control by an individual with tetraplegia." The Lancet 381.9866 (2013): 557-564.
O’Doherty, Joseph E., et al. "Active tactile exploration using a brain–machine–brain interface." Nature 479.7372 (2011): 228.
Flesher, Sharlene N., et al. "Intracortical microstimulation of human somatosensory cortex." Science translational medicine8.361 (2016): 361ra141-361ra141.
Ghez, C., et al. "Roles of proprioceptive input in the programming of arm trajectories." Cold Spring Harbor symposia on quantitative biology. Vol. 55. Cold Spring Harbor Laboratory Press, 1990.