Probing Somatosensory Representations in the Cuneate Nucleus of Awake Primates
Sliman Bensmaia, Department of Organismal Biology and Anatomy
University of Chicago
People: Chris Versteeg and Josie Wallner
The brainstem cuneate nucleus (CN) receives ascending input from somatosensory afferents that innervate the skin, joints, and muscles as well as descending input from sensorimotor cortex. While the tactile and proprioceptive response properties of primary afferents and of neurons in primary somatosensory cortex (S1) have been extensively characterized, virtually nothing is known of the response properties of CN neurons. Multiple types of cutaneous afferents innervate the glabrous skin of the hand and play different, albeit overlapping roles in tactile perception. Receptors in muscles and tendons provide signals related to muscle length, length change, and force. These sensors have strong context dependent firing that is modulated by attention and phases of movement, but such context-sensitivity is not seen at the cortical level. We have begun for the first time, with our colleagues at the University of Chicago, to characterize the response properties of CN neurons in awake monkeys and to assess (1) the degree to which signals from different somatosensory submodalities converge onto single CN neurons; (2) the extent to which the feature selectivity observed in S1 begins to emerge in CN; (3) the degree to which responses recorded in CN depend on the context of the arm movement they reflect. We anticipate that this study will have important implications for our understanding of somatosensory processing and could even inform the development of subcortical brain-machine interfaces used to restore somatosensation in persons with spinal cord injury.
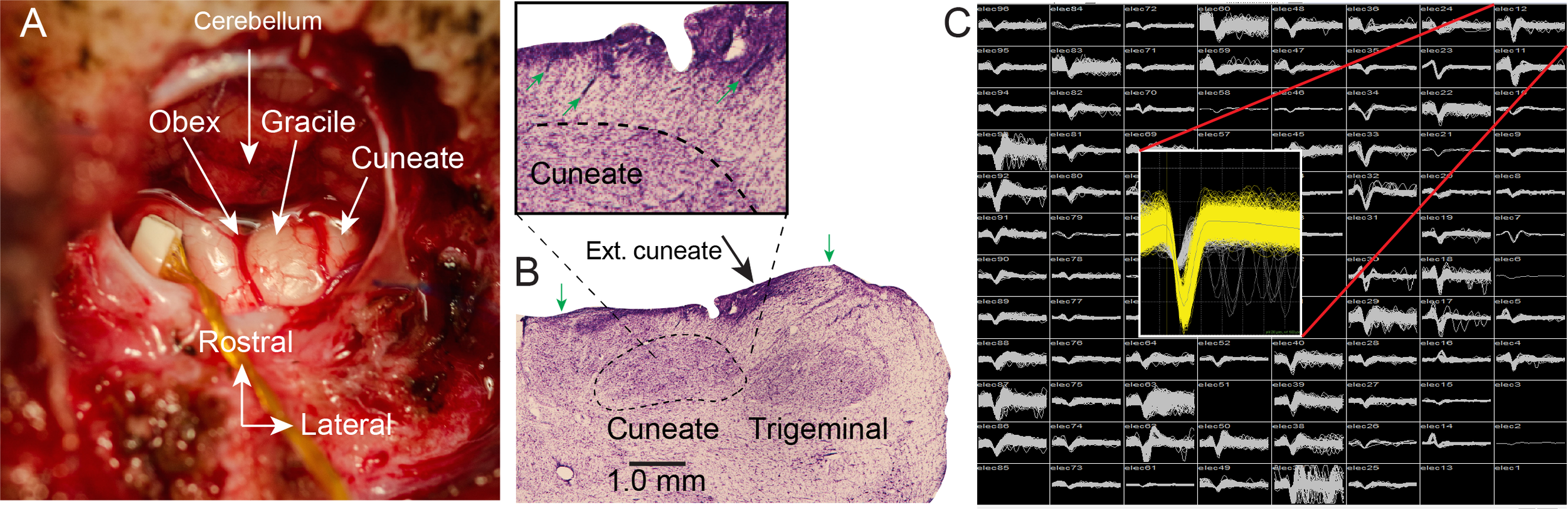
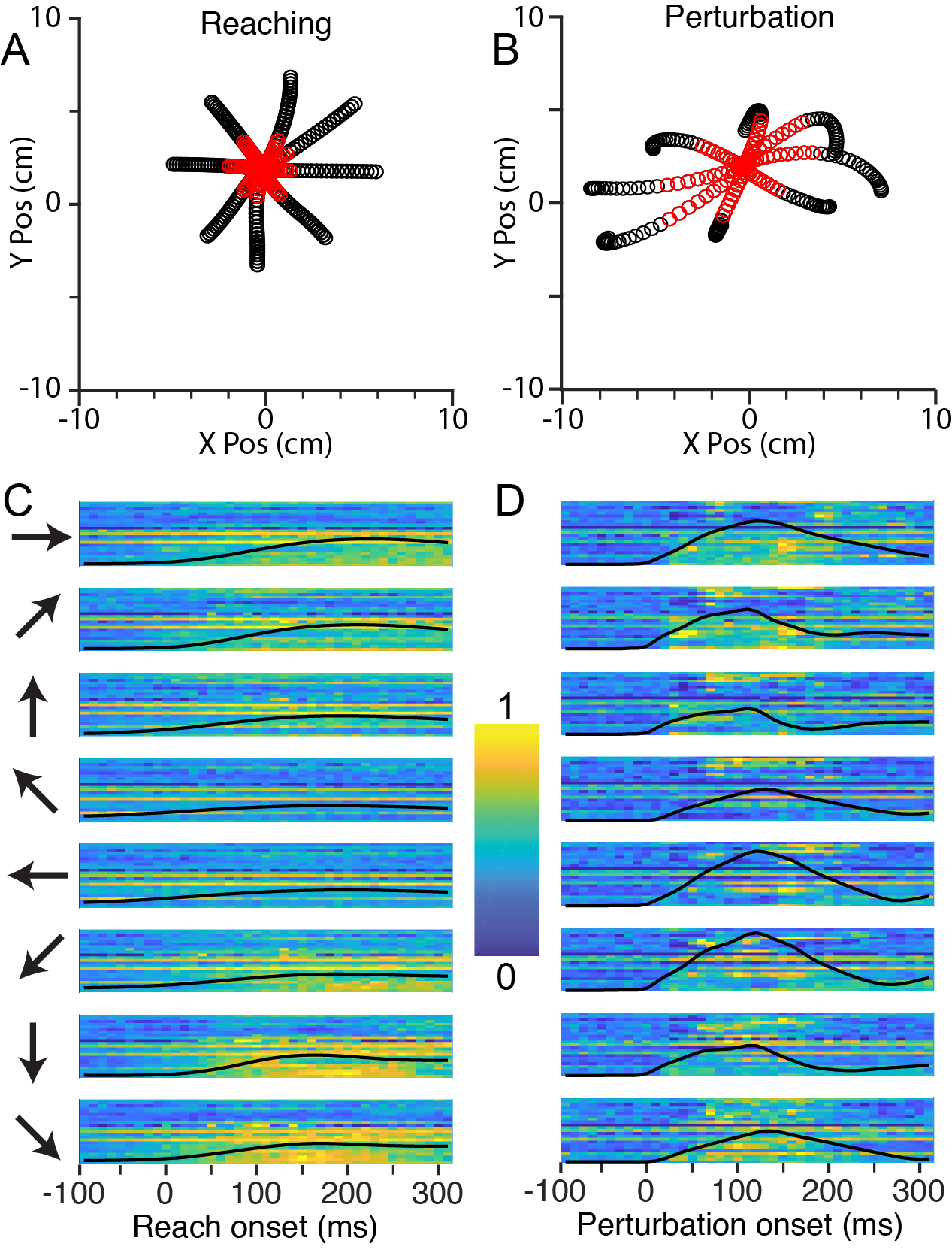
We have implanted three monkeys with multi-electrode arrays in the CN, including a Floating Microelectrode Array (Microprobes for Life Sciences) which we used in one of the initial monkeys (Fig 1A). Histology performed on one monkey after experiments were complete, revealed that the array, in this case, a Utah Electrode Array (Blackrock Microsystems) had been placed in the main cuneate nucleus (Fig 1B). Figure 1C shows typical recordings from one of the successful implants. The monkeys performed a center-out reaching task controlling a robotic manipulandum with the hand ipsilateral to the recording electrodes (Fig 2A). On half of the trials, when the monkey moved the cursor to the center of the workspace, the robot bumped the monkey’s hand, which generated a passive movement similar to the actively generated reaches (Fig 2B). We recorded neurons in CN with either muscle and cutaneous receptive fields, which were activated during both active and passive movements (Fig 2C,D). To quantify their tuning properties, we fit Poisson Generalized Linear Models (GLMs) to the firing rates of these neurons as a function of hand velocity, from which we calculated the preferred direction of each neuron during active and passive movements. Many of these neurons had strong preferences in their firing rates for movement in specific directions, similar to those previously reported from primary motor and somatosensory areas. We found a significant non-uniformity in the distribution of PDs, with a large proportion of neurons firing maximally when the hand moved towards the body (Fig 3A, top row). Moving from CN to cortex, the number of muscle receptors represented in single neurons appears to increase, which we infer through an increase in the uniformity of the PD distributions. The distribution in CN resembles that of modeled neurons receiving input from only one muscle, and is somewhat less uniform than that of area 2 (fig 3A top right plot).
The brainstem has recently become a focus for attention as a source of fast feedback control signals, which have been shown to be adaptive and context dependent. Fast, task-dependent feedback corrections of movement errors may require a proprioceptive system that flexibly modulates sensory gains. We are working now to better explain the firing rates of neurons in CN by comparing them to features of the muscle spindle discharge to determine how descending drive is modulated to enable these corrective actions. Understanding the proprioceptive encoding along the neuraxis will help elucidate the connections between the motor system and the feedback which is critical to its operation.
Fig 1: Electrode arrays implanted in dorsal brainstem yield single neuron recordings from the cuneate nucleus: A- Intraoperative exposure of the dorsal brainstem and cuneate nucleus following implantation of an FMA. The obex and cerebellar tonsils are in the center of the image. Gracile nucleus is the structure immediately lateral to the midline, with the main CN further lateral. B - Histological examinations of Nissl-stained sections in one monkey showed that the implant successfully targeted the main CN. The boundaries of the CN and Trigeminal nuclei are well delineated. Main CN begins at ~0.5 mm depth and extends to ~2 mm. External cuneate is more lateral and shallower. Trigeminal nucleus is further lateral. Green arrows mark the mediolateral limits of the array. Inset shows an enlargement of the area surrounding CN. Green arrows mark several electrode tracks leading into the main CN.
Fig 2: CN activity during Center-Out reaching and limb perturbation A: X/Y plot of mean handle position during reaching (-100 ms to +300 ms from movement onset), averaged across ~120 trials per direction. Red symbols are from 0 ms to 130 ms. B: Corresponding plot during perturbation trials. Significant mechanical anisotropy can be seen due to the non-uniform impedance of the hand. C: Neural firing rates during reaching in 8 directions, indicated by the arrows to the left of the plots. Each row of pixels represents a single CN neuron, with color indicating the normalized firing rate. The black line superimposed on the image is the speed of the hand, normalized to the fastest hand speed in both the active and perturbation conditions. D: Firing rates during perturbation trials, as in panel C.
Fig 3: Directional tuning in cuneate nucleus and somatosensory cortex. A – Neurons in CN have similar reaching and perturbation preferred directions. Each point represents a single CN neuron that was sinusoidally tuned in both the reaching and perturbation conditions. The x and y-axes denote the PDs during reaching and perturbation, respectively. The red dashed line indicates the unity line. The diameter of each circle is proportional to the max tuning depths in reaching and perturbation and the error bars denote the bootstrapped 95% confidence interval on the PD of each neuron in each condition. B - CN neurons have strong biases in their preferred direction distributions. Each column denotes a single monkey; the top row shows the distribution of reach PDs, the bottom row, perturbation PDs. The number of neurons is shown in the top right of each plot. C – Same format for B, except with area 2 neurons for two additional monkeys.